About Molnupiravir
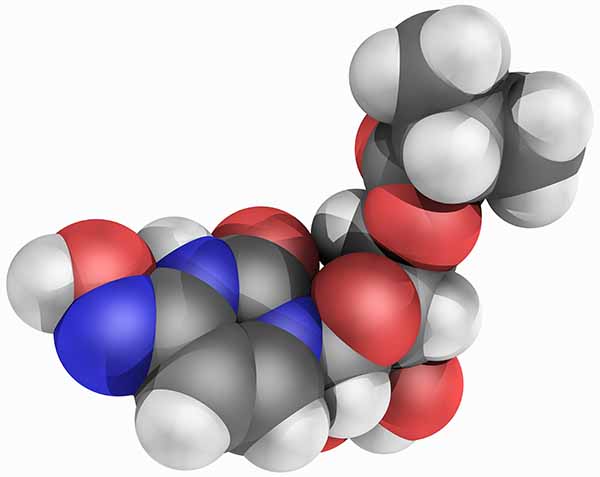
Molnupiravir was invented at Emory University. Drug Innovation Ventures at Emory (DRIVE) LLC, which was formed by Emory to develop early-stage drug candidates for viral diseases of global concern, advanced molnupiravir through an Investigational New Drug submission. On Dec. 23, 2021, molnupiravir received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the treatment of COVID-19.
Background
For several years, Emory scientists had been working on EIDD-2801, a drug candidate with potential as a treatment for several highly infectious and worrisome viral diseases, including influenza and Venezuelan Equine Encephalitis virus, some of which are biodefense threats. When the pandemic began, the team quickly turned its attention to developing EIDD-2801 as a treatment for COVID-19. DRIVE licensed EIDD-2801, now known as molnupiravir, to Ridgeback Biotherapeutics in 2020, which conducted the first human clinical trials and then partnered with Merck.
How does molnupiravir work?
Molnupiravir disrupts the process by which SARS-CoV-2 duplicates itself in host cells by inserting errors into the virus’s genetic code.
What does its name mean?
The name molnupiravir refers to the hammer—Mjölnir—of the Norse god and Marvel superhero Thor. The suffix “vir” is frequently used in naming antiviral drugs.
Timeline
- 2013
Equine Encephalitis
Emory begins screening ribonucleoside analogs against equine encephalitis viruses (EEVs). Spread by mosquitoes, EEVs can be fatal and were weaponized during the Cold War.
- 2016
Emory receives Defense Threat Reduction Agency contract to develop countermeasures against EEVs.
- 2019
Emory receives NIH award to develop antivirals against influenza.
- March 2020
COVID-19
DRIVE licenses EIDD-2801 to Ridgeback Biotherapeutics as a potential COVID-19 therapy.
- April 2020
The FDA grants DRIVE an Investigational New Drug application. Ridgeback begins Phase 1 clinical trials.
- May 2020
Ridgeback partners with Merck.
- September 2020
Merck and Ridgeback begin pivotal Phase 2/3 clinical trial.
- October 2021
Merck submits an Emergency Use Authorization application to the FDA.
- November 2021
Britain becomes the first country to approve molnupiravir for COVID-19.
- December 2021
Molnupiravir receives Emergency Use Authorization from the FDA for the treatment of COVID-19.
